Ph: 02 4089 3744
Position your trial for success.
Novatrials.
The smart choice.
The numbers you need to know
>1.2M
geographical population; median age >40, 84% born in Australia, 4-5% identifying as Aboriginal and/or Torres Strait Islander
<5min
Time taken to drive from facility to contracted emergency/ICU departments
3x
Number of University campuses in region for Phase I healthy studies
43.5%
Australia's R&D tax incentive (rebate)
THE SMART CHOICE FOR CLINICAL TRIALS
We work with you to ensure we meet all expectations!
01
High quality service in a state-of-the-art facility
Using the latest in online technology, our clinical and quality systems for managing patients and clinical trials are fully cloud integrated, supporting virtual monitoring and compliant to ICH-GCP, ISO9001 and 21 CFR Part-11 standards.
02
Exceeding client expectations
As an organisation built on agile work practices, we rapidly respond to change, with fast turnaround times. We pride ourselves on surpassing performance targets (such as recruitment) without compromising on quality.
03
Value for money
Located in a large regional metropolitan centre, close to a major capital city, our reduced operating costs are passed onto our clients while our proximity to urban infrastructure (e.g. airports) supports logistics and monitoring efficiencies.
04
Patient-orientated
As testament to living our values (Respect, Integrity, Teamwork and Excellence), through offering a welcoming and friendly environment, our participants consistently report >99% positive feedback in our participant surveys. Our ethnically diverse community are referred to as a demographic microcosm of Australia.
05
Unique geographical location
For over 60 years the Hunter has been used for trialling new products and services, seen as a demographic microcosm of Australia. Its people have an open attitude to 'trying something new' with word of mouth a strong communication tool. These characteristics along with low traffic congestion and only a two hours drive from Sydney for monitoring, make Newcastle a perfect location for conducting trials.
06
Effective recruitment and retention strategies
Exceeding recruitment targets, we are regularly the highest recruiting site in Australia (even globally). Our success is due to our marketing and advertising expertise focussed on bespoke trial-specific strategies. Our dedicated recruitment team manage a recruitment databases of >10k, as well as targeted media advertising, community engagement and referrals from other health practitioners. Our comprehensive pre-screening process is designed to eliminate costly screen failures.
Staff are always amazing! Always caring, happy and full of laughs, I love coming here!
Don't change anything, its perfect!
Anonymous Participant Feedback (2024)
Our Services
Our team of medical and clinical experts manage and coordinate in-patient and out-patient services through our purpose-built, state-of-the-art, clinical trial facility.
Phase I Clinical Trials
Headed by our Medical Director (and Clinical Pharmacologist) Prof. Jennifer Martin, our custom-built Phase I unit comprises of a 30-bed in-patient facility, state-of-the-art, cutting edge technology and designer recreational amenities.
Pre-study support
We offer services to help you get your study off the ground from protocol review and feedback, to feasibility assessments and budget estimations. Our wide range of on-hand clinical specialists provide advice across all a range of therapeutic areas.
Study recruitment & coordination
Our systems support rapid study start-up and recruitment. As lead site, we manage ethics and regulatory processes and our dedicated recruitment team manage pre-screening through a database of >10,000 people. Out-patient and in-patient trials are coordinated via 8 consultation rooms, 30-bed clinic and isolation ward.
Onsite pharmacy & laboratory
Our onsite pharmacy means all IMP is managed securely close to patient, stored in locked refrigerators or ambient cabinets depending on requirements. Our pharmacy staff are experienced in dispensing medications and maintaining IP accountability. Our onsite processing laboratory houses fridges, -20C and -80C freezers and refrigerated/ambient centrifuges.
Our Experience
Novatrials has been engaged by major pharmaceutical, biotechnology, academic and CROs (clinical research organisations) to conduct Phase I – IV trials.
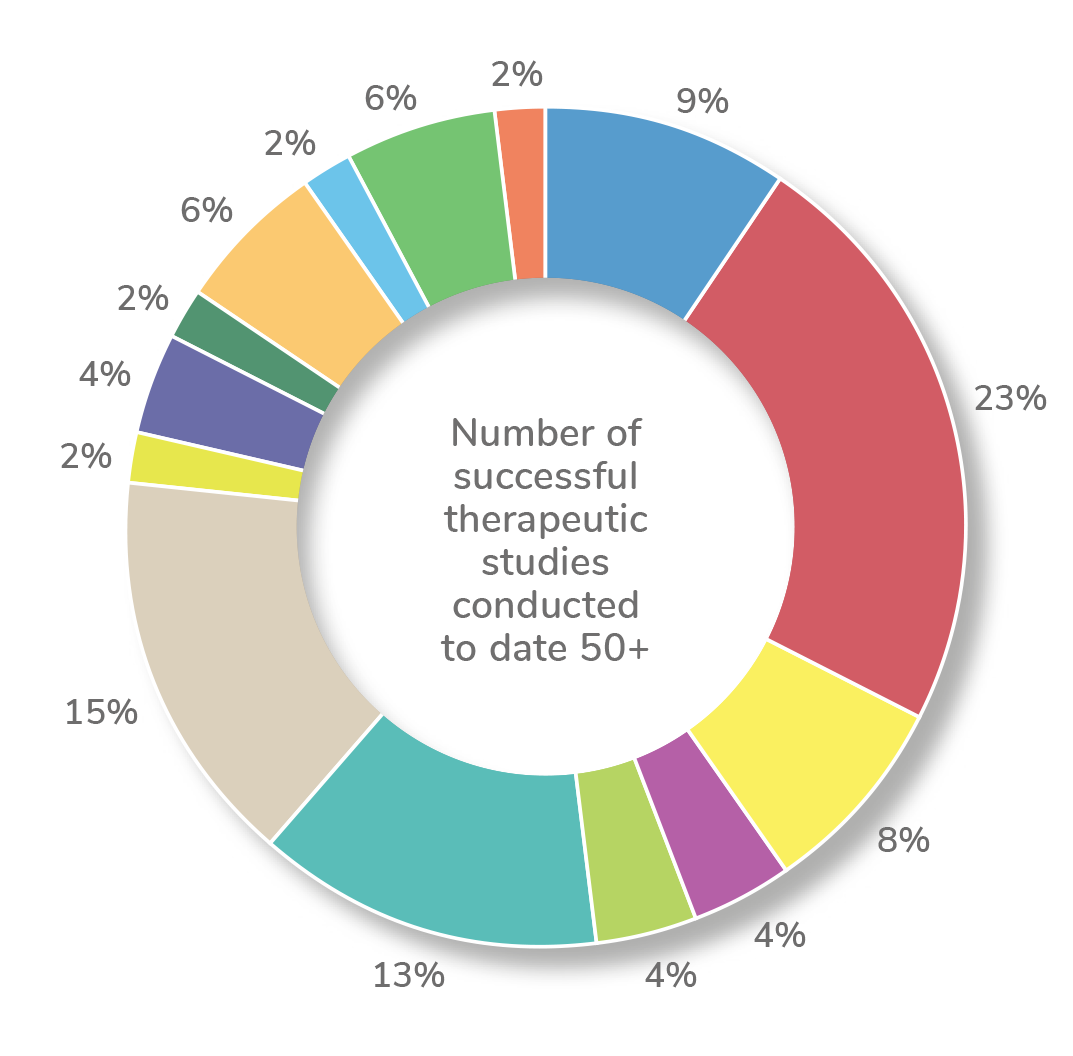
Our experience has been across a wide range of therapeutic areas including: cardiology, dermatology, endocrinology, infectious diseases, metabolic, musculoskeletal, neurology, oncology, pain management, rheumatology, urology, vaccines, women's health.